
1

2

3

4

5

6

7
8
9

10
11

12

13

14

15

16

17
18

19
20

21

22

23
4 Good morning. Welcome to our virtual attendees and those on the call-in line. And, welcome to our board of directors, company principals and consultants online today. My name is Andrew Dahl, president and chief executive officer of ZIVO Bioscience Incorporated, a fully-reporting public company trading in the OTC market.
5 Before proceeding further, I must advise you that this report and any Exhibits to it contain forward-looking statements that involve risks and uncertainties. These statements reflect the Company’s future plans, objectives, expectations and intentions, and the assumptions underlying or relating to any of these statements. These statements may be identified by the use of the words “anticipate,” “expect,” “estimate,” “intend,” “believe,” and similar expressions. The Company’s actual results could differ materially from those discussed in these statements. Factors that could contribute to these differences include, but are not limited to, those discussed in this report.
6 In the 17 months since our last shareholder meeting, the Company has continued to focus on capital funding activities for biotech and agtech research and product development, compliance and building out a global supply chain for algae production. Since the beginning of 2020, the Company was able to attract roughly $4.2 million in new funding, which allowed us to make significant progress toward our objectives, and which I am happy to share with you today.
For those of you who may not be familiar with ZIVO Bioscience, the Company holds novel intellectual property in the form of biologically active molecules and compounds, patented applications and processes, an optimized algal culture and nutritional products derived from our proprietary algal biomass that can find their way into food, feed, supplements and potentially therapeutics.
We operate in two sectors – biotech and agtech. The biotech sector is focused on developing biologically active molecules as additives or therapeutics, while agtech is focused on our developing proprietary algae as a nutritional product, including cultivation, processing and marketing as a functional food ingredient, dietary supplement and skin health product, among many such applications and market verticals.
7 ZIVO Bioscience conducts much of its lab and clinical research at universities and private research organizations across the US. Beginning in early spring, the COVID pandemic forced the closure of many academic research departments, as well as partial or complete shutdown of private facilities, including ours. Fortunately, despite these delays, we were able to keep moving forward, albeit at a slower pace.
8 I’m especially heartened by the progress we’ve made over the last 18 months in validating our claims relative to poultry gut health and the attention that this has drawn to our company and our research. We’ve recently concluded our 16th trial at an independent poultry research facility in Maryland, and the results again point to the potential to positively impact feed conversion ratios and enhance immune response to invasive pathogens. For those of you not familiar with raising broiler chickens at commercial scale, the feed conversion ratio, or FCR, is a measure by which poultry producers can assess how much their birds grow in relation to the feed consumed. Because of the vast scale of production, even very small improvements translate into significant returns for the producer. Also, cutting back on medicated feeds or antimicrobials typically mixed into chicken feed is both environmentally and economically beneficial. In fact, there is increasing demand for poultry products that contain NO traces of antibiotics, antimicrobials, chemicals or hormones. We believe our timing is quite good in this respect.
Coccidiosis, or inflammation of the intestinal tract, remains one of the leading health concerns in the global poultry industry. We have previously announced results from more than a dozen poultry studies that provide evidence of the benefits of ZIVO’s active compounds in minimizing the negative effects of coccidiosis on growth performance, intestinal morphology, and other measures of digestive health in broilers. Each of these previous studies employed coccidiosis testing protocols common to the industry, intended to mimic naturally acquired coccidiosis in many respects and represents the industry’s best experimental tool for evaluating new ways to combat the devastating effects of coccidiosis.
9 Today, I very pleased to announce the results from two additional poultry studies performed at separate research facilities in which ZIVO’s active compounds were shown to provide clear benefit to broiler flocks exposed to coccidiosis pathogens already in litter from previous flocks occupying the same house, as that exposure to these pathogens may occur in a production environment. In the first of these studies, we significantly enhanced growth performance compared to the control group with a 3% improvement in the Feed Conversion Ratio (FCR) over the 42-day grow out period. A similar improvement of just under 3% was observed in the second study. Again, given the large scale and extremely tight margins under which poultry producers operate, these consistent improvements translate into significant cost savings.
These findings suggest that ZIVO products may be used as growth promoters in healthy birds as well as birds affected by clinically obvious coccidiosis. Moreover, these findings are consistent with results of previous immune and metabolic pathway profiling work done by the company that has indicated that ZIVO products may enhance growth and potentially accelerate development of a robust innate and acquired immune response.
24
10 With respect to invasive pathogens in broilers, you may remember a December 2019 announcement made regarding a novel molecule and its ability to modulate immune response – essentially mobilizing the immune system yet holding down the inflammatory response which would typically accompany such an immune response. We filed a patent application then and have since filed almost a dozen more. And now, 10 months later, we’ve completed pilot-scale production of that novel polysaccharide molecule in fermenter/digester vessels at two university labs and two commercial labs. We are making this product candidate in much the same way that insulin or an enzyme would generally be created. It represents a clean break from our proprietary ZIVO algal culture which originally spawned it. We believe this production method will be more attractive to licensees, as the fermenter/digestor production model is well-established and commonplace in the food and biopharmaceutical industries.
11 I’m also happy to report today that our most recent poultry tests reveal that we’re also making headway against common poultry gut bacteria that, while not necessarily harmful to chickens in all cases, are dangerous to humans – namely, salmonella and campylobacter, both of which are significantly reduced or eliminated when our product was mixed into chicken feed. We have results compiled over several studies that demonstrate the reduction of campylobacter, as well as a reduction of salmonella, e. coli and clostridium perfringens in the gut. So, in addition to the health benefits for the birds themselves, the reduction of pathogen loads may positively impact food safety in poultry processing plants and ultimately consumers handling raw poultry at home. All, without an overreliance on antibiotics or antimicrobials.
12 With the study findings and production method in hand, we are now fully endeavoring to establish a strategic partnership with a global animal health company. In order to fund this initiative, we offered a special licensing co-development deal to new and existing shareholders over the last 10 months to raise the $3 million we estimated would be needed to put a poultry deal in front of a prospective global partner. This special funding was closed out about 2 weeks ago.
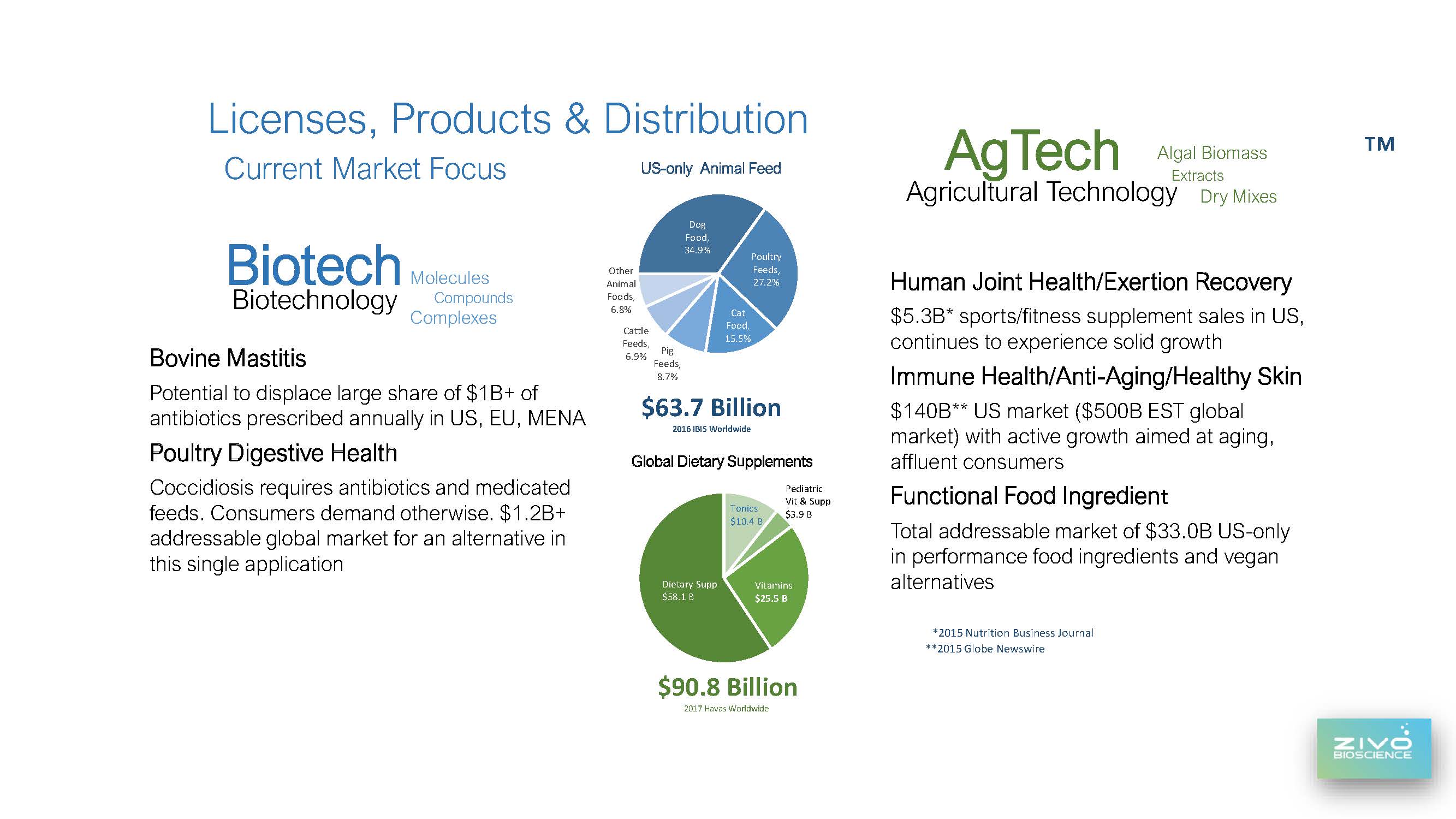
We believe the poultry market is an exceptional opportunity for ZIVO. According to the 2020 annual Alltech feed survey, broilers consume more than 51 million metric tons of feed in North America alone, and we are ranked fourth in global poultry production. If our product candidate is mixed in at a ratio of 100 grams or roughly 4 ounces per ton of chicken feed, we would be looking at a potential market our product in the thousands of tons per year in North America alone.
13 As many of you know, our research has also focused on bovine mastitis, an inflammation of the udder that impacts milk production and as you’ve heard me mention on many occasions, is responsible for nearly $3 billion in annual losses to US dairy producers alone, and billions more worldwide. As a point of reference, the US dairy herd of 9 million represents less than 3% of the world’s 244 million dairy cows.
The bovine mastitis research has been moving toward its final phase of validation for nearly 2-1/2 years, as we overcame challenges ranging from sample quality to funding shortfalls, to the availability of scientists to conduct field studies and analytics in what is a very specialized field of research. Over that same span of time, we have sponsored or engaged academic researchers from a dozen universities and independent researchers or contract research organizations in the US, U.K. and France. In late 2019, we commenced another mastitis study with our long-time collaborator Dairy Experts, based in the Central Valley of northern California.
The study itself was delayed due to funding and the prioritization of the poultry R&D effort, driven by the remarkable results and positive reception from the poultry industry. Given our limited resources, we were still able to finish a small bovine mastitis study, and then analyze the findings over several months when time permitted. We came away with confirmation of previous studies and some interesting results consistent with the poultry research running at the same time.
Firstly, milk returned to normal faster in the ZIVO treated group, measured as fewer days with abnormal milk. By normal, we mean milk appearance, bacterial count and similar quality and safety measures. This corroborates previous results and points to the advantages of using the ZIVO product. Fewer days of abnormal milk means more days of marketable milk. And much like in the poultry studies, we observed systemic effects, where the counts of aerobic bacteria such as staph and strep were reduced significantly in all teats as compared to untreated control groups. By significantly, we mean statistically significant. This again promotes food safety and may lower costs to the producers.
This bovine study commenced before we had fully isolated and validated our molecular entity as announced last December. Instead this study used extracts from the algal culture rather than the purified molecular entity produced by the fermenter digester method. Although these are largely one and the same, we will be re-running the study with the purified molecular entity before offering the study findings to a potential licensee. The reason for the additional study is that we increase the value of the license by offering a purified, fully characterized molecular entity along with the methods to manufacture it at scale and a new safety profile, as well.
25
What I want to add here is that we have, since the isolation and characterization of our novel molecule late last year, completely refocused our biotech research away from our proprietary algal culture or extracts thereof and toward the isolated, biologically active molecules which we are now producing in bioreactors or fermenter/digester vessels by a completely different method. This is critically important, because these new developments represent a watershed for the Company’s R&D efforts, with the goal of a potential license or joint development agreement.
Therefore, our biotech research has now pivoted toward these active molecular entities, the optimization of these molecules and their means of production.
14 That doesn’t mean we walk away from years of R&D work associated with the algae. Far from it. In fact, that work is accelerating, as well. We have touted the nutritional and functional benefits of ZIVO algae for years and it’s not necessary to repeat them here. However, there have been some interesting developments on the algae or agtech side of the business.
In March of 2020, ZIVO launched a modest product development program to determine whether the whole dried algal biomass held any promise as a functional skin health topical product. Preliminary tests indicate that incorporating ZIVO algal biomass into topical treatments may provide positive benefits. The Company has engaged a formulations lab to create a product concept utilizing ZIVO algae. However, COVID-related delays have pushed back the delivery date and subsequent PET safety testing to assure safe use as a topical skin care product. We resumed the formulation process and safety testing to resume in late September. Once approved, ZIVO algae can be offered for sale in the US as a topical ingredient for skin health.
You may remember that ZIVO had entered into a letter of intent with Grekin Laboratories to create a line of nutricosmetics, which are ingested and not applied topically. That proposed joint venture is still on the table, pending the availability of ZIVO algal biomass at commercial scale. In fact, whether ingested or applied topically, the success of ZIVO agtech rests on bringing algae production up to commercial scale.
15 Earlier this year, after more than a year of planning and negotiation, ZIVO signed a licensing & supply agreement and a phased production contract with Peruvian agribusiness Grupo Alimenta, which operates an algae production facility in that country. The agreement calls for significant expansion of the Alimenta facility outside Ica, and the installation of post-processing and packaging equipment, which has already begun. Alimenta has significant experience and capability in algal cultivation and was recently awarded the Rockefeller Green Prize in recognition of its sustainability efforts across all of its operations. Production has commenced, and we are expecting saleable biomass in the next few months.
Z-Mexico Farms, an American company based near Aguacalientes, Mexico has entered into negotiations with ZIVO to build an algae production facility at its own expense and license the ZIVO algal culture and production methods. In support of that opportunity, ZIVO has committed engineering and production resources. We have retained a civil engineering firm to design the ponds and processing systems, fluid dynamics to model efficient waterflow, as well as cultivation expertise to develop more efficient production methods and practices. To that end, Dr. Harlan Miller joined the Company as Vice-President, Technology & Global Supply. Dr. Miller will head up all algal production, technology transfer, global supply logistics and assumes P&L responsibility for all agtech activities. By way of background, Dr. Miller has nearly 15 years’ experience in cleantech, agtech, and biotech industries and continues to be dedicated to developing environmentally responsible technologies and sustainable products. He leads a growing team of scientists and consultants to support the Mexican and Peruvian growers in their scaleup efforts, as well as our sole contract grower in India, previously Shibin, now renamed ALG.
The Z-Mex facility is being built specifically with the intention of growing, harvesting and processing ZIVO algae at commercial scale. We expect the existing Alimenta plant in Peru is to be expanded to included purpose-built ponds and water handling optimized for ZIVO algae, pending available funding and/or licensing agreements we close in the meantime. We also continue our efforts to recruit existing algae growers around the world, at the moment focused on the Americas and India.
16 Simultaneously, Dr. Miller has launched an IP integrity initiative, bringing to the ZIVO lab in Plymouth, Michigan all the various strains of ZIVO algae and symbionts that have resided within laboratories at Arizona State, National Center for Marine Algae and Microbiota, University of Texas, University of Georgia and elsewhere for the past few years. The pedigree and parent stocks for inoculum production will be maintained and protected at the Plymouth, Michigan lab for the foreseeable future. We will still maintain frozen samples at the Bigelow Lab in Maine and at the algae repository at the University of Texas.
A larger experimental lab is planned for some time in 2021, to be located in southern Florida. Here, ZIVO researchers intend to optimize various cultivation strategies, production enhancements and develop new intellectual property revolving around process and yield improvements. We will continue to produce algal biomass at Arizona State for the time being, and Dr. Valerie Harmon will continue to produce ZIVO inoculum at her lab in Hawaii.
26
Therefore, on the agtech side of the business, the R&D shifts from “does it work” and “is it safe” to “how do we grow it efficiently” and “how do we improve the bottom line” for ZIVO and its licensed growers. So, more “D” for practical development, and less “R” or basic research.
17 The agtech side of the business also includes product development work for the algal biomass, be it human or animal applications. For example, one of our most recent experiments is the extraction of valuable non-starch polysaccharides from the algal biomass to use as active ingredients for topical skin health applications, and as dietary supplement ingredients, followed by FDA compliance testing. By doing so, we can offer to potential licensees a premier functional ingredient, with test data in hand.
Whether as a functional food ingredient, a skin health product, a dietary supplement or nutricosmetic ingredient, this product development work will continue. One of the defining differences between ZIVO and other algae species and other algae producers is that we are committed to exploring various applications of ZIVO algal culture that can generate attention and even create new, exclusive product categories.
Continuing this development work provides us with the data, formulations and product concepts to approach licensees in various market verticals, such as anti-aging, joint health and skin health, along with vegan and vegetarian dietary ingredients. These licensees would likely welcome the food science, safety claims and formulations we’ve developed. As we’ve stated previously, our goal is not to create a consumer brand or sell directly to consumers. Instead, we aim to align ourselves with larger, well-established brand names in nutrition and immune health where we are positioned as the premier functional ingredient.
18 We have, after all these years, worked to place ourselves in a position to offer licensed products, licensed distribution and licensed applications for our agtech portfolio as our biomass production comes online.
In summary, ZIVO principals have been working in two separate tracks, agtech and biotech – food and pharma, to create intellectual property that can be licensed or sold. We work across two very different industry sectors, each with its own timetable, expertise and funding demands. We remain focused on a select few key opportunities, each holding breakout potential.
19 As stated previously, the Company has no internal testing facilities, no lab or clinical staff. William Pfund, our Vice-President of R&D, works closely with our longtime Director of R&D, Dr. Amy Steffek, to manage a wide-ranging portfolio of experiments and studies. They are now joined by Dr. Harlan Miller and Casey Fowler to expand and protect the intellectual property portfolio. All R&D work is conducted by independent labs, contract research organizations, academic institutions, specialty firms or independent researchers.
In managing this matrix approach, the money, the schedules and the deliverables may line up perfectly. Other times, they do not. We’ve been able to recruit top researchers in highly specialized fields of study, protect our IP and adjust to the timeframe available.
20 The advantage we have over a good many biotech and agtech companies is that we’re not married to a single product, a single course of action, a particular field of science, or even the expertise of one individual. Our work in immune health bridges biotech and agtech.
21 Concluding ZIVO core business, we now address Wellmetrix LLC, a wholly owned subsidiary of ZIVO focused on monitoring health and wellness. Given all of the activity surrounding ZIVO and the pressure on available resources, the board of directors has concluded that it is in the best interests of all stakeholders that Wellmetrix is shelved for the time being, the only activity being the protection of intellectual property in the form of patents pending.
If you haven’t already done so, please subscribe to our email blast, which occurs roughly every 2 months, and updates ZIVO stakeholders on various projects and developments.
21 And with that, I conclude my report to shareholders and wish everyone safe and healthy holidays, as we look forward to a better new year.
I’d like to invite our chief financial officer, Philip Rice to join me. We’ll begin taking questions from attendees. Thank you.
27